Procedure: New Market Authorization Class I-IV
Country: Global Procedure
Version #: 01/08/2024
Author: Daria Kostiuchenko
Editor: Daria Kostiuchenko
ID: GP-MD-VI-IV-SR
Category: Medical Devices
Validity: For five years
Certified by: Vlad Reznikov
Copyright: Pattern of USA Inc.
SUMMARY
The classification of medical devices into Class I to IV is commonly used in regulatory frameworks in various countries and regions. The classification is based on the level of risk associated with the device, with Class I representing the lowest risk and Class IV the highest. Here’s a general overview of where the classification system is implemented in some ML4 counties:
1. United States (FDA): The U.S. Food and Drug Administration (FDA) classifies medical devices into three categories – Class I, Class II, and Class III. Class I devices are considered low risk, Class II devices are moderate risk, and Class III devices are high risk. The classification determines the regulatory requirements for market authorization, with Class III devices requiring the most stringent review process.
2. European Union (EU): In the EU, medical devices are classified into four classes – Class I, Class IIa, Class IIb, and Class III. The classification is based on the potential risks associated with the device. Class I devices are considered low risk, while Class III devices pose the highest risk. The classification determines the conformity assessment route for market authorization, with higher-risk devices requiring involvement from a Notified Body.
3. Canada: Health Canada classifies medical devices into four classes – Class I, Class II, Class III, and Class IV. The classification is based on the risk associated with the device and the regulatory controls required. Class I devices are considered low risk, while Class IV devices pose the highest risk. The classification determines the regulatory requirements for market authorization, with Class IV devices requiring the most stringent review process.
4. Australia: The Therapeutic Goods Administration (TGA) in Australia classifies medical devices into four classes – Class I, Class IIa, Class IIb, and Class III. The classification is based on the risk associated with the device. Class I devices are considered low risk, while Class III devices pose the highest risk. The classification determines the regulatory requirements for market authorization, with higher-risk devices requiring involvement from a Conformity Assessment Body.
5. Japan: In Japan, medical devices are classified into three classes – Class I, Class II, and Class III. The classification is based on the risk associated with the device. Class I devices are considered low risk, while Class III devices pose the highest risk. The classification determines the regulatory requirements for market authorization, with Class III devices requiring the most stringent review process.
Must-Have Requirements, Recognized Standards, and Certifications:
- Device description, including variants
- Technical documentation (MDR/IVDR)
- Instructions for use (IFU)
- Quality Management System (QMS/QSR)
- Good Manufacturing Practice (GMP)
- Declaration of Conformity (DoC)
- Risk Management
- Clinical Evidence
- POA
- Substantially equivalent to the predicate device
- MAs in other countries
- CE mark and ISO 13485 in many countries
Nice-to-have:
- IEC 60601
- ISO 14971
- ISO 10993
- Labeling
- Regulatory Compliance and Gap Analysis see GP-PhR-GAP-SR card
- Regulatory Intelligence and Strategic Consulting see GP-PhR-RI-SR card
- Q-Sub Activities see GP-MD-QSA-SR card
Typical Gaps and Deficiencies: Understanding of the regulatory pathway and requirements applicable to the device. Incorrect predicate device selection. Inadequate documentation. Rush in submitting the application without being prepared
Implementation Period: Immediately upon approval
Deliverables: Market Authorization (Clearance or Registration), NRA Listing and Establishment registration
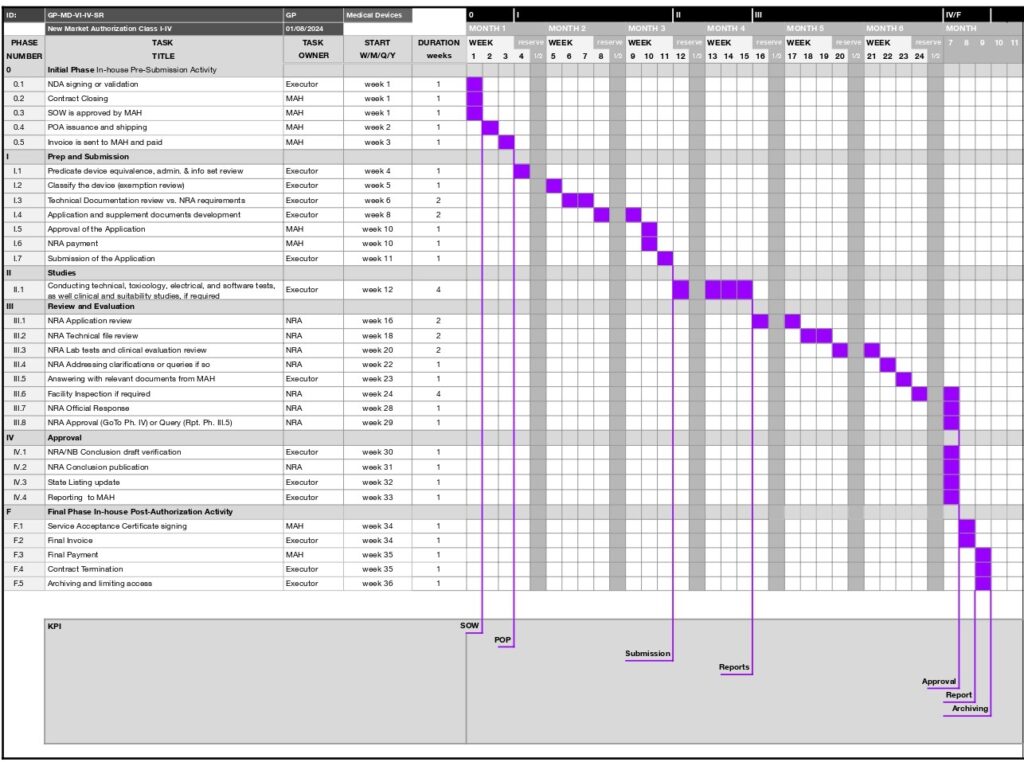
PHASE | TASK | TASK | START | DURATION | Fee | KPI | ||
NUMBER | TITLE | OWNER | W/M/Q/Y | in weeks | 3rd Party | Service | State | |
0 | In-house Pre-Submission Activity | |||||||
0.1 | NDA signing or validation | Executor | week 1 | 1 | ||||
0.2 | Contract Closing | MAH | week 1 | 1 | ||||
0.3 | SOW is approved by MAH | MAH | week 1 | 1 | SOW | |||
0.4 | POA issuance and shipping | MAH | week 2 | 1 | ||||
0.5 | Invoice is sent to MAH and paid | MAH | week 3 | 1 | POP | |||
I | Prep and Submission | |||||||
I.1 | Predicate device equivalence, admin. & info set review | Executor | week 4 | 1 | ||||
I.2 | Classify the device (exemption review) | Executor | week 5 | 1 | ||||
I.3 | Technical Documentation review vs. NRA requirements | Executor | week 6 | 2 | ||||
I.4 | Application and supplement documents development | Executor | week 8 | 2 | ||||
I.5 | Approval of the Application | MAH | week 10 | 1 | ||||
I.6 | NRA payment | MAH | week 10 | 1 | ||||
I.7 | Submission of the Application | Executor | week 11 | 1 | Submission | |||
II | Studies | |||||||
II.1 | Сonducting technical, toxicology, electrical, and software tests, as well clinical and suitability studies, if required | Executor | week 12 | 4 | Reports | |||
III | Review and Evaluation | |||||||
III.1 | NRA Application review | NRA | week 16 | 2 | ||||
III.2 | NRA Technical file review | NRA | week 18 | 2 | ||||
III.3 | NRA Lab tests and clinical evaluation review | NRA | week 20 | 2 | ||||
III.4 | NRA Addressing clarifications or queries if so | NRA | week 22 | 1 | ||||
III.5 | Answering with relevant documents from MAH | Executor | week 23 | 1 | ||||
III.6 | Facility Inspection if required | NRA | week 24 | 4 | ||||
III.7 | NRA Official Response | NRA | week 28 | 1 | ||||
III.8 | NRA Approval (GoTo Ph. IV) or Query (Rpt. Ph. III.5) | NRA | week 29 | 1 | Approval | |||
IV | Approval | |||||||
IV.1 | NRA/NB Conclusion draft verification | Executor | week 30 | 1 | ||||
IV.2 | NRA Conclusion publication | NRA | week 31 | 1 | ||||
IV.3 | State Listing update | Executor | week 32 | 1 | ||||
IV.4 | Reporting to MAH | Executor | week 33 | 1 | Report | |||
F | Final Phase In-house Post-Authorization Activity | |||||||
F.1 | Service Acceptance Certificate signing | MAH | week 34 | 1 | ||||
F.2 | Final Invoice | Executor | week 34 | 1 | ||||
F.3 | Final Payment | MAH | week 35 | 1 | ||||
F.4 | Contract Termination | Executor | week 35 | 1 | ||||
F.5 | Archiving and limiting access | Executor | week 36 | 1 | Archiving |
Feel free to submit a Request for Proposal (RFP) for a specific country or territory to info@patternofusa.com
