Procedure: REMS and ETASU Development
Country: United States
Version #: 01/05/2024
Author: Vlad Reznikov
Editor: Daria Kostiuchenko
ID: US-PhR-RMS-SR
Category: Pharmaceuticals
Validity: Next FDA call for action
Certified by: Vlad Reznikov
Copyright: Pattern of USA Inc.
SUMMARY
The FDA may require Risk Evaluation and Mitigation Strategies (REMS) and Elements to Assure Safe Use (ETASU) for drugs with specific safety concerns. REMS and ETASU are designed to identify, mitigate, and manage risks associated with certain pharmaceutical products. They help ensure that the benefits of the drug outweigh potential risks, contributing to overall patient safety. While ensuring patient safety, REMS and ETASU also aim to avoid unnecessary restrictions that could hinder patient access to essential medications. The creation of Risk Evaluation and Mitigation Strategies (REMS) and Elements to Assure Safe Use (ETASU) can take three forms: De Novo, Single-Shared System (SSS), and Updates and Modifications. Balancing safety measures with the need for timely access is a key consideration in the development of these strategies. These risk management strategies often involve communication and educational efforts directed at healthcare providers, patients, and other stakeholders. The development of REMS and ETASU involves collaboration with the FDA to align risk management efforts with regulatory expectations. This collaborative approach helps foster transparency and compliance with regulatory requirements. A comprehensive development approach involves Knowledge, Attitude, and Behavior (KAB) testing. The timeline and KPI for development varies depending on the type and extent of the work undertaken.
Must-Have Requirements, Recognized Standards, and Certification:
- Risk Assessment
- Benefit-Risk Analysis
- FDA Request or Mandate
- IND; NDA, BLA, or ANDA MA Application
Nice-to-have:
- Risk Mitigation Plan (RMP), aRMM
- State-specific list of Healthcare Providers&Establ.
Typical Gaps and Deficiencies: Lack of time, incomplete communication system with healthcare establishments, IT issues
Implementation Period: Upon FDA approval
Deliverables: digital safety monitoring system, educational programs, restricted distribution plan, healthcare provider training materials, patient education info, prescribing restrictions, including, but not limited to:
- Medication guide for patients, Black box warning
- Communication plan for healthcare professionals
- DHCP Letter
- Implementation Plan
- Monitoring and Assessment
- Data Collection systems
- Reporting Requirements
- Documentation kit for FDA, etc.
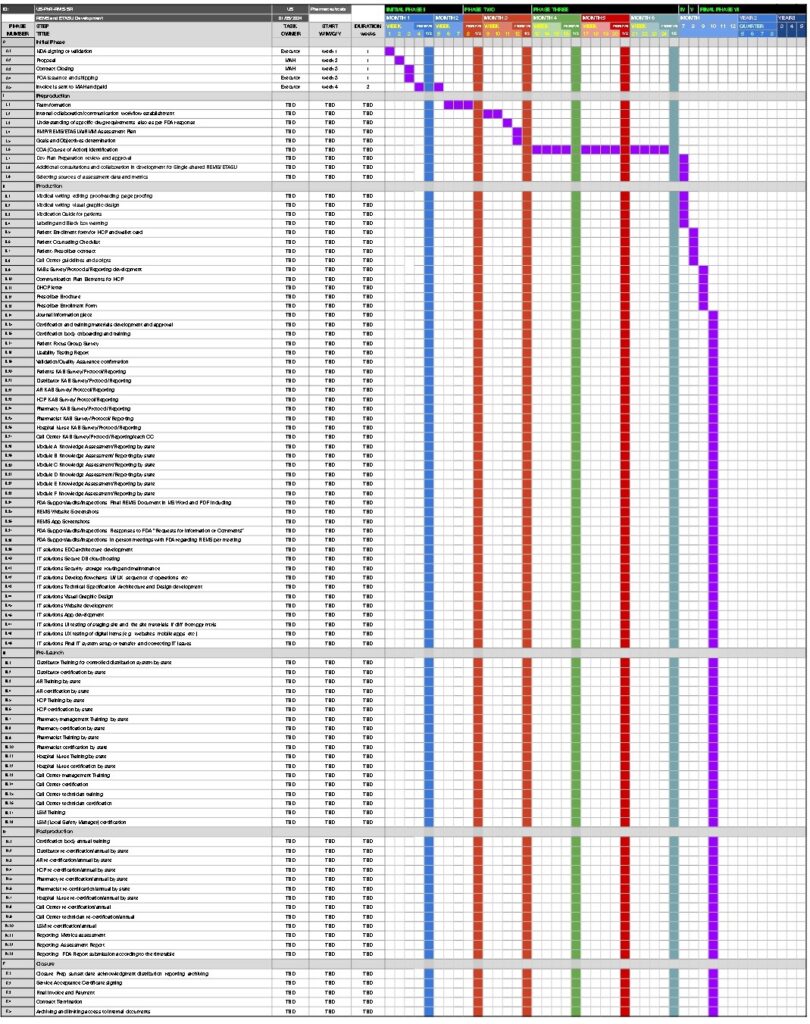
| PHASE | TASK | TASK | START | DURATION | Fee | KPI | ||
| NUMBER | TITLE | OWNER | W/M/Q/Y | in weeks | 3rd Party | Service | State | |
| 0 | Initial Phase | |||||||
| 0.1 | NDA signing or validation | Executor | week 1 | 1 | ||||
| 0.2 | Proposal | MAH | week 2 | 1 | ||||
| 0.3 | Contract Closing | MAH | week 3 | 1 | ||||
| 0.4 | POA issuance and shipping | Executor | week 3 | 1 | ||||
| 0.5 | Invoice is sent to MAH and paid | Executor | week 4 | 2 | ||||
| I | Preproduction | |||||||
| I.1 | Team formation | TBD | TBD | TBD | ||||
| I.2 | Internal collaboration/communication, workflow establishment | TBD | TBD | TBD | ||||
| I.3 | Understanding of specific drug requirements per FDA response | TBD | TBD | TBD | ||||
| I.4 | RMP/REMS/ETASU/aRMM Assessment Plan | TBD | TBD | TBD | ||||
| I.5 | Goals and Objectives determination | TBD | TBD | TBD | ||||
| I.6 | COA (Course of Action) identification | TBD | TBD | TBD | ||||
| I.7 | Dev Plan Preparation, review, and approval | TBD | TBD | TBD | ||||
| I.8 | Additional consultations and collaboration in development for Single-shared REMS/ETASU | TBD | TBD | TBD | ||||
| I.9 | Selecting sources of assessment data and metrics | TBD | TBD | TBD | ||||
| II | Production | |||||||
| II.1 | Medical writing: editing, proofreading, page proofing | TBD | TBD | TBD | ||||
| II.2 | Medical writing: visual graphic design | TBD | TBD | TBD | ||||
| II.3 | Medication Guide for patients | TBD | TBD | TBD | ||||
| II.4 | Labeling and Black box warning | TBD | TBD | TBD | ||||
| II.5 | Patient Enrollment form for HCP and wallet card | TBD | TBD | TBD | ||||
| II.6 | Patient Counseling Checklist | TBD | TBD | TBD | ||||
| II.7 | Patient-Prescriber contract | TBD | TBD | TBD | ||||
| II.8 | Call Center guidelines and scripts | TBD | TBD | TBD | ||||
| II.9 | KABs Survey/Protocols/Reporting development | TBD | TBD | TBD | ||||
| II.10 | Communication Plan Elements for HCP | TBD | TBD | TBD | ||||
| II.11 | DHCP letter | TBD | TBD | TBD | ||||
| II.12 | Prescriber Brochure | TBD | TBD | TBD | ||||
| II.13 | Prescriber Enrollment Form | TBD | TBD | TBD | ||||
| II.14 | Journal information piece | TBD | TBD | TBD | ||||
| II.15 | Certification and training materials development and approval | TBD | TBD | TBD | ||||
| II.16 | Certification body onboarding and training | TBD | TBD | TBD | ||||
| II.17 | Patient Focus Group Survey | TBD | TBD | TBD | ||||
| II.18 | Usability Testing Report | TBD | TBD | TBD | ||||
| II.19 | Validation/Quality Assurance confirmation | TBD | TBD | TBD | ||||
| II.20 | Patients KAB Survey/Protocol/Reporting | TBD | TBD | TBD | ||||
| II.21 | Distributor KAB Survey/Protocol/Reporting | TBD | TBD | TBD | ||||
| II.22 | AR KAB Survey/Protocol/Reporting | TBD | TBD | TBD | ||||
| II.23 | HCP KAB Survey/Protocol/Reporting | TBD | TBD | TBD | ||||
| II.24 | Pharmacy KAB Survey/Protocol/Reporting | TBD | TBD | TBD | ||||
| II.25 | Pharmacist KAB Survey/Protocol/Reporting | TBD | TBD | TBD | ||||
| II.26 | Hospital Nurse KAB Survey/Protocol/Reporting | TBD | TBD | TBD | ||||
| II.27 | Call Center KAB Survey/Protocol/Reporting/each CC | TBD | TBD | TBD | ||||
| II.28 | Module A Knowledge Assessment/Reporting by state | TBD | TBD | TBD | ||||
| II.29 | Module B Knowledge Assessment/Reporting by state | TBD | TBD | TBD | ||||
| II.30 | Module C Knowledge Assessment/Reporting by state | TBD | TBD | TBD | ||||
| II.31 | Module D Knowledge Assessment/Reporting by state | TBD | TBD | TBD | ||||
| II.32 | Module E Knowledge Assessment/Reporting by state | TBD | TBD | TBD | ||||
| II.33 | Module F Knowledge Assessment/Reporting by state | TBD | TBD | TBD | ||||
| II.34 | FDA Support/audits/inspections: Final REMS Document in MS Word and PDF, including | TBD | TBD | TBD | ||||
| II.35 | REMS Website Screenshots | TBD | TBD | TBD | ||||
| II.36 | REMS App Screenshots | TBD | TBD | TBD | ||||
| II.37 | FDA Support/audits/inspections: Responses to FDA “Requests for Information or Comments” | TBD | TBD | TBD | ||||
| II.38 | FDA Support/audits/inspections: In-person meetings with FDA regarding REMS per meeting | TBD | TBD | TBD | ||||
| II.39 | IT solutions EDC architecture development | TBD | TBD | TBD | ||||
| II.40 | IT solutions Secure DB cloud hosting | TBD | TBD | TBD | ||||
| II.41 | IT solutions Security, storage, routing and maintenance | TBD | TBD | TBD | ||||
| II.42 | IT solutions Develop flowcharts, UI/UX, sequence of operations, e | TBD | TBD | TBD | ||||
| II.43 | IT solutions Technical Specification, Architecture and Design development | TBD | TBD | TBD | ||||
| II.44 | IT solutions Visual Graphic Design | TBD | TBD | TBD | ||||
| II.45 | IT solutions Website development | TBD | TBD | TBD | ||||
| II.46 | IT solutions App development | TBD | TBD | TBD | ||||
| II.47 | IT solutions UI testing of staging site and, the site materials, if diff. | TBD | TBD | TBD | ||||
| II.48 | IT solutions UX testing of digital items (web, mobile apps, etc.) | TBD | TBD | TBD | ||||
| II.49 | IT solutions Final IT system setup or transfer, and fixing IT issues | TBD | TBD | TBD | ||||
| III | Pre-Launch | |||||||
| III.1 | Distributor Training for controlled distribution system by state | TBD | TBD | TBD | ||||
| III.2 | Distributor certification by state | TBD | TBD | TBD | ||||
| III.3 | AR Training by state | TBD | TBD | TBD | ||||
| III.4 | AR certification by state | TBD | TBD | TBD | ||||
| III.5 | HCP Training by state | TBD | TBD | TBD | ||||
| III.6 | HCP certification by state | TBD | TBD | TBD | ||||
| III.7 | Pharmacy management Training by state | TBD | TBD | TBD | ||||
| III.8 | Pharmacy certification by state | TBD | TBD | TBD | ||||
| III.9 | Pharmacist Training by state | TBD | TBD | TBD | ||||
| III.10 | Pharmacist certification by state | TBD | TBD | TBD | ||||
| III.11 | Hospital Nurse Training by state | TBD | TBD | TBD | ||||
| III.12 | Hospital Nurse certification by state | TBD | TBD | TBD | ||||
| III.13 | Call Center management Training | TBD | TBD | TBD | ||||
| III.14 | Call Center certification | TBD | TBD | TBD | ||||
| III.15 | Call Center technician training | TBD | TBD | TBD | ||||
| III.16 | Call Center technician certification | TBD | TBD | TBD | ||||
| III.17 | LSM Training | TBD | TBD | TBD | ||||
| III.18 | LSM (Local Safety Manager) certification | TBD | TBD | TBD | ||||
| IV | Postproduction | |||||||
| IV.1 | Certification body annual training | TBD | TBD | TBD | ||||
| IV.2 | Distributor re-certification/annual by state | TBD | TBD | TBD | ||||
| IV.3 | AR re-certification/annual by state | TBD | TBD | TBD | ||||
| IV.4 | HCP re-certification/annual by state | TBD | TBD | TBD | ||||
| IV.5 | Pharmacy re-certification/annual by state | TBD | TBD | TBD | ||||
| IV.6 | Pharmacist re-certification/annual by state | TBD | TBD | TBD | ||||
| IV.7 | Hospital Nurse re-certification/annual by state | TBD | TBD | TBD | ||||
| IV.8 | Call Center re-certification/annual | TBD | TBD | TBD | ||||
| IV.9 | Call Center technician re-certification/annual | TBD | TBD | TBD | ||||
| IV.10 | LSM re-certification/annual | TBD | TBD | TBD | ||||
| IV.11 | Reporting: Metrics assessment | TBD | TBD | TBD | ||||
| IV.12 | Reporting: Assessment Report | TBD | TBD | TBD | ||||
| IV.13 | Reporting: FDA Report submission according to the timetable | TBD | TBD | TBD | ||||
| F | Closure | |||||||
| F.1 | Prep, sunset date, acknowledgment distribution, reporting, archive | TBD | TBD | TBD | ||||
| F.2 | Service Acceptance Certificate signing | TBD | TBD | TBD | ||||
| F.3 | Final Invoice and Payment | TBD | TBD | TBD | ||||
| F.4 | Contract Termination | TBD | TBD | TBD | ||||
| F.5 | Archiving and limiting access to internal documents | TBD | TBD | TBD |
Feel free to submit a Request for Proposal (RFP) for a specific country or territory to info@patternofusa.com
