Procedure: Type IA and IA-IN variation notifications
Country: Global Procedure
Version #: 01/03/2024
Author: Mariya Ivanova
Editor: Daria Kostiuchenko
ID: GP-PhR-VIA-SR
Category: Pharmaceuticals
Validity: Until next MA expiration or update
Certified by: Vlad Reznikov
Copyright: Pattern of USA Inc.
SUMMARY
Type-IA variations are minor variations that have only a minimal impact or no impact at all, on the quality, safety or efficacy of the medicinal product, and do not require prior approval before implementation (‘do-and-tell’ procedure). The Classification Guideline clarifies the conditions that must be met for a change to be considered a type-IA variation.
Such minor variations are classified in two subcategories, which impact on their submission:
- IAIN Type-IA variations requiring immediate notification
The Classification Guideline specifies the type-IA variations that must be notified (submitted) immediately to the NRA or EMA following implementation, in order to ensure the continuous supervision of the medicinal product.
- IA Type-IA variations not requiring immediate notification
Variations that do not require immediate notification may be submitted by the MAH within 12 months after implementation, or may be submitted earlier should this facilitate dossier lifecycle maintenance or when necessary, to ensure that the latest product information is reflected in certificates of pharmaceutical products, for example.
Must-Have Requirements, Recognized Standards, and Certification:
- Annex A Comparative Table
- Annex IV Elements
- Grouping of Type IA/IAIN variations
- Applicable SmPC or Annexes I, II, IIIA/B
Nice-to-have:
- Confirmation of the original variation
- Pre-notification checklist
Typical Gaps and Deficiencies: Non-eCTD, Insufficient regulatory documentation, confusing dossier presentation
Implementation Period: Immediately or up to a 12-month period, also with a new batch, defined by MAH and NRA
Deliverables: Confirmation of the MA terms adjustment
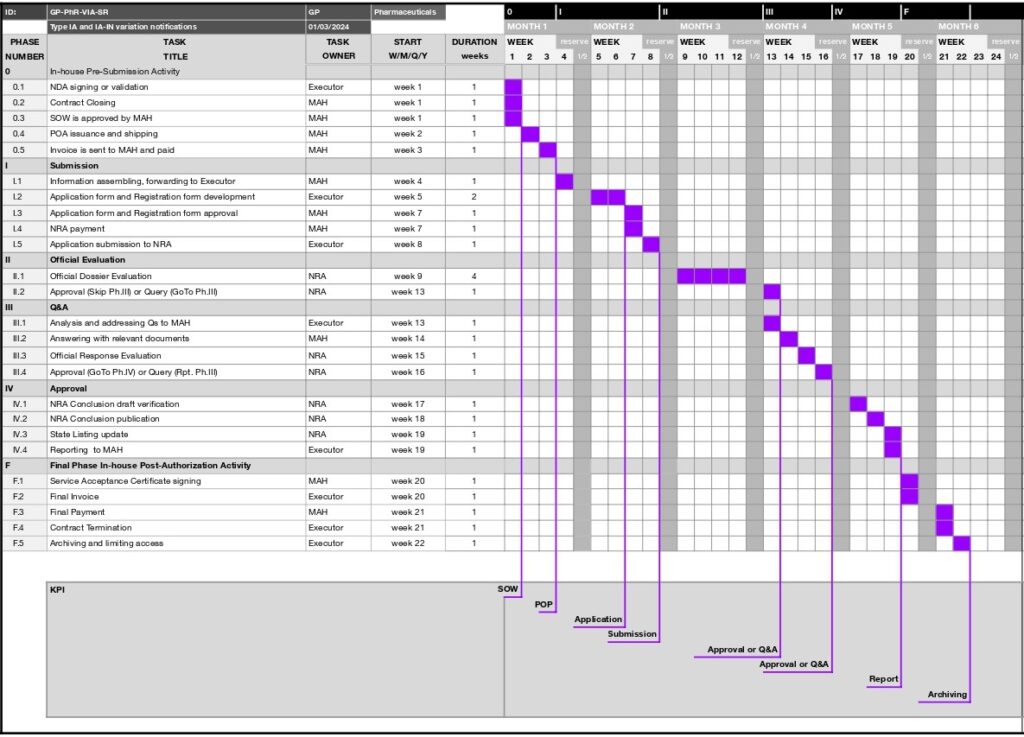
PHASE | TASK | TASK | START | DURATION | Fee | KPI | ||
NUMBER | TITLE | OWNER | W/M/Q/Y | in weeks | 3rd Party | Service | State | |
0 | In-house Pre-Submission Activity | |||||||
0.1 | NDA signing or validation | Executor | week 1 | 1 | ||||
0.2 | Contract Closing | MAH | week 1 | 1 | ||||
0.3 | SOW is approved by MAH | MAH | week 1 | 1 | SOW | |||
0.4 | POA issuance and shipping | MAH | week 2 | 1 | ||||
0.5 | Invoice is sent to MAH and paid | MAH | week 3 | 1 | POP | |||
I | Submission | |||||||
I.1 | Information assembling, forwarding to Executor | MAH | week 4 | 1 | ||||
I.2 | Application form and Registration form development | Executor | week 5 | 2 | Application | |||
I.3 | Application form and Registration form approval | MAH | week 7 | 1 | ||||
I.4 | NRA payment | MAH | week 7 | 1 | ||||
I.5 | Application submission to NRA | Executor | week 8 | 1 | Submission | |||
II | Official Evaluation | |||||||
II.1 | Official Dossier Evaluation | NRA | week 9 | 4 | ||||
II.2 | Approval (Skip Ph.III) or Query (GoTo Ph.III) | NRA | week 13 | 1 | Approval or Q&A | |||
III | Q&A | |||||||
III.1 | Analysis and addressing Qs to MAH | Executor | week 13 | 1 | ||||
III.2 | Answering with relevant documents | MAH | week 14 | 1 | ||||
III.3 | Official Response Evaluation | NRA | week 15 | 1 | ||||
III.4 | Approval (GoTo Ph.IV) or Query (Rpt. Ph.III) | NRA | week 16 | 1 | Approval or Q&A | |||
IV | Approval | |||||||
IV.1 | NRA Conclusion draft verification | NRA | week 17 | 1 | ||||
IV.2 | NRA Conclusion publication | NRA | week 18 | 1 | ||||
IV.3 | State Listing update | NRA | week 19 | 1 | ||||
IV.4 | Reporting to MAH | Executor | week 19 | 1 | Report | |||
F | Final Phase In-house Post-Authorization Activity | |||||||
F.1 | Service Acceptance Certificate signing | MAH | week 20 | 1 | ||||
F.2 | Final Invoice | Executor | week 20 | 1 | ||||
F.3 | Final Payment | MAH | week 21 | 1 | ||||
F.4 | Contract Termination | Executor | week 21 | 1 | ||||
F.5 | Archiving and limiting access | Executor | week 22 | 1 | Archiving |
Feel free to submit a Request for Proposal (RFP) for a specific country or territory to info@patternofusa.com
