Over twenty years ago, Muse headbands began as an experiment in neurofeedback: The gadget notified the wearer when an electroencephalography (EEG) signal from their brain reached a certain threshold. As the product developed, its maker, Toronto-based Interaxon, began marketing the device as a meditation tool.
Although mindfulness tech remains part of the Muse brand, the potential scope of its wearable devices has now expanded into sleep and performance. The Muse S Athena headband, announced earlier this year, is the first device from the company to also incorporate light sensors able to measure blood oxygenation; the system uses fluctuations in that data as an indicator of blood flow and brain activity. This technique, called functional near-infrared spectroscopy, or fNIRS, is considered complementary to EEG.
In June, Muse also announced a reworked data analysis tool that the company calls a “foundation brain model.” The AI model has a transformer architecture, similar to that of ChatGPT, and it was trained on over a billion minutes of brain data collected over the past decade from Muse users.
Though the headbands are designed as consumer products and the company makes no claims about diagnostic ability, previous Muse headbands have been used for a range of scientific studies, from exploring its therapeutic applications to basic behavioral research. The company hopes this one will be too. Researchers familiar with fNIRS and EEG recording told IEEE Spectrum that the device has advantages in portability, affordability, and ease of use, but noted that its sensor limitations could preclude its use for some research questions.
What can brain researchers do with a Muse headband?
“We’re focused on bringing neurotechnology to the home,” says Chris Aimone, chief innovation officer and co-founder of Interaxon. He says many of Interaxon’s research partners are interested sleep science, and notes that the S Athena can be used as an at-home sleep monitor—its measurements of both brain activity and blood oxygenation could be useful for assessing disorders such as sleep apnea. An earlier version of the Muse S performed comparably to standard laboratory sleep testing equipment.
The Muse S Athena is not the only commercialized brain monitor to integrate fNIRS and EEG sensors, but it may occupy a niche in the tradeoff between coverage and cost. It has four EEG sensors placed along the brow line, five light sensors for fNIRS measurements on the forehead, and costs around US $500 with additional subscription fees for some services. In comparison, a full-coverage helmet from Kernel, which boasts fNIRS and EEG sensors that can provide up to 3,500 measurement channels, costs more than $100,000.
So what do researchers think of the gear? The Muse systems “have limited channels [and] very limited capability for imaging,” says Hubin Zhao, an engineer at University College London who works in neuromedical technologies. “If you have more sensors—more measurement channels—you get richer information with better resolution.”
“You have to really plan your study accordingly, knowing that you may only have info from these few frontal sensors,” says Guiomar Niso, a neuroscientist at the Cajal Institute, in Madrid, who has written about other wearable EEG systems.
Aimone says that rather than pinpointing brain activity, the S Athena records an “interesting macroscopic signal.”
 The Muse S Athena headband uses two kinds of sensors to measure brain activity. Muse
The Muse S Athena headband uses two kinds of sensors to measure brain activity. Muse
“As a consumer-oriented portable device, it may present some challenges regarding data quality and artifact removal compared to more sensitive laboratory-grade systems,” says Mevhibe Saricaoğlu, a neuroscience Ph.D. student at Istanbul Medipol University who has used concurrent fNIRS and EEG recordings to compare cognitive performance across age groups. However, “the device’s practicality and ease of use offer significant advantages for field studies, neurofeedback applications, and preliminary pilot experiments.”
A device designed for everyday use could translate well to studies of everyday activities. “If you do research and you’re stuck in a lab, you’re limiting the kind of questions you can ask,” says Olav Krigolson, a neuroscientist at the University of Victoria, in Canada, who has worked extensively with previous generations of Muse devices. For example, he monitored the brains of entire classrooms of high school students and studied mental fatigue in both emergency room workers and pilots in their natural settings.
What’s next for Muse
Muse headbands could further expand the scope of research through a new citizen science program that allows users to enroll in individual studies through an app.
A similar approach has enabled Muse to collect what Aimone calls “an enormous pile of data,” which is critical for training a successful AI model. “You need to train something that has the complexity of a brain to understand one,” says Aimone. “We’re not doing that yet, but in some ways that’s the goal: Something that has the expressive power to identify brain patterns and functions, and also differences in brain patterns and functions between people.”
The overall package of device, software and services “marks a shift from isolated physiological tracking toward integrated, AI-enhanced brain monitoring,” says Saricaoğlu. “Muse S Athena and similar systems are powerful tools—but they must be used ethically, transparently, and with a full awareness of both their capabilities and their limitations.”














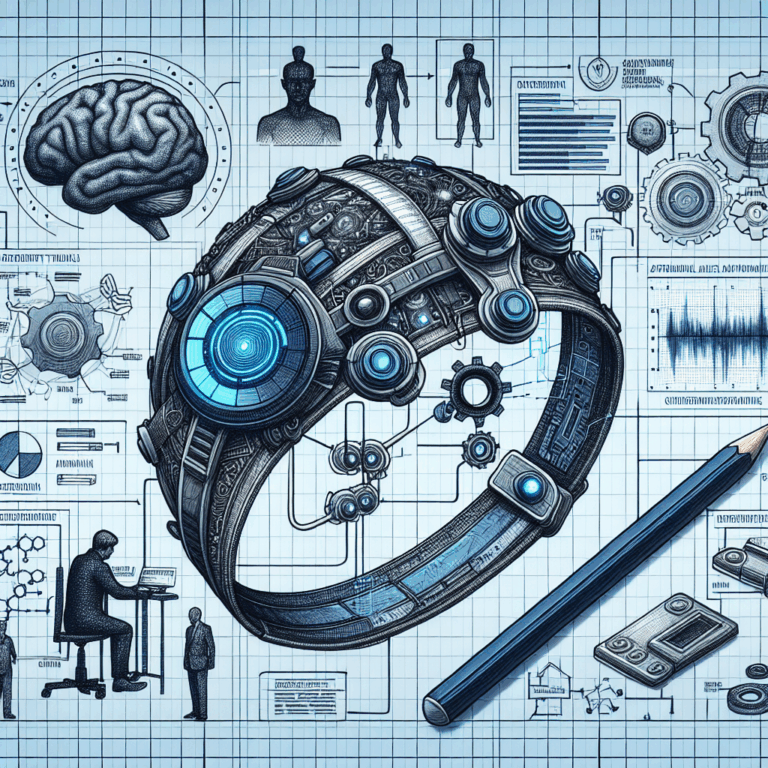

 The Muse S Athena headband uses two kinds of sensors to measure brain activity. Muse
The Muse S Athena headband uses two kinds of sensors to measure brain activity. Muse