
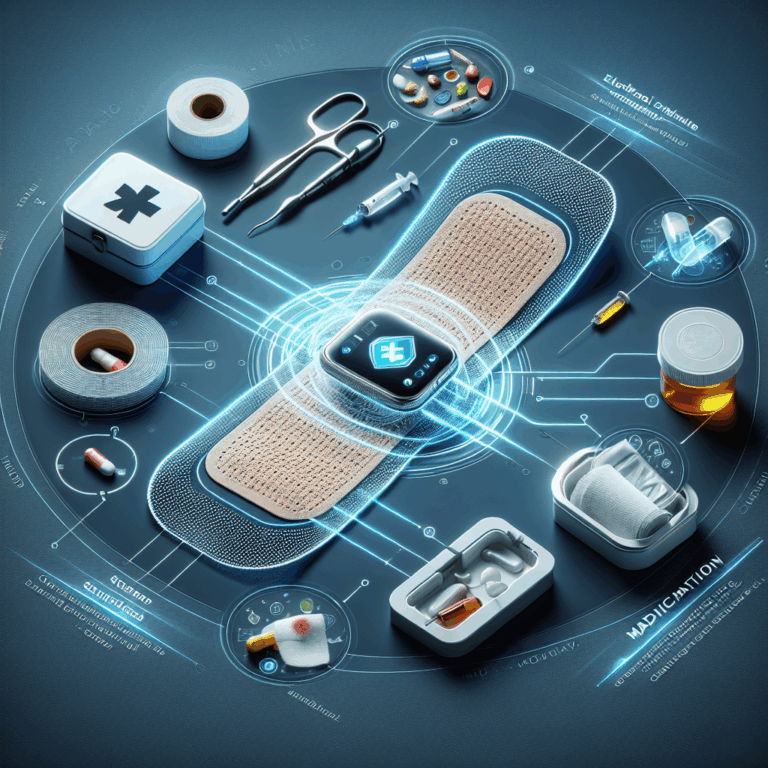
A smart bandage could speed up wound healing by actively tracking and responding to the healing process. The proof-of-concept device, called a-Heal, was designed to fit inside a commercial colostomy bandage and contains a camera that takes images of the wound every two hours, as well as a wireless connection to a machine learning module that provides updated recommendations on how to stimulate healing.
A multidisciplinary research team led by Marco Rolandi, an electrical engineering and computer science professor at the University of California Santa Cruz, developed a-Heal. Rolandi says the initial motivation was to reduce healing time for battlefield injuries by 50 percent as part of a Defense Advanced Research Projects Agency initiative.
While a-Heal was tested on a wound (on a pig) that was caused by injury and can heal quickly, Rolandi says it can be applied to other types of wounds that have difficulty healing—such as chronic wounds or ones that become infected.
Current wound dressings are a one-size-fits-all approach that can’t account for variations in how individual wounds heal.
To heal wounds faster, a-Heal monitors wounds, diagnoses the current healing stage of the wound, suggests treatments, and delivers those treatments. Through repeating the process, the bandage creates a feedback control mechanism.
Rolandi says that a machine learning algorithm, ML Physician, analyzes each image of the wound, “compares it with training data, and decides at what stage the wound is at and whether the wound is healing in the desired fashion, or whether the wound requires an intervention or a treatment to speed up the healing.” Based on the AI recommendation, the wearable activates bioelectronic actuators to deliver one of two treatments, both known to increase healing in animals: electrically stimulating the wound to reduce inflammation or infusing it with fluoxetine, a drug that promotes tissue growth.
The researchers tested the device in a pig because a pig’s skin is in many ways similar to human skin, says Min Zhao, a physician and professor of dermatology at University of California Davis Health.
Although bandages offering electrical stimulation already exist, a-Heal goes a few steps further. “I’m not aware of somebody having used photographic information in combination with a kind of closed loop actuation system. So it’s definitely combining two things that people are working on in a new way,” says Geoffrey Gurtner, a physician, surgeon, and professor at the University of Arizona with a joint appointment at Stanford University, who was not involved in the study.
How the Smart Bandage Heals Wounds Faster
Every two hours, the bandage’s camera module takes 11 images at different focal depths. When asked how well the camera could image darker human skin tones, Rolandi says that if training data exists, it should be okay, but it’s too soon to tell because this version of the device was not intended for human trials.
The ML Physician machine learning algorithm, located on a laptop, implements a leader-follower strategy to analyze the images. A portion of the algorithm, Deep Mapper, generates a predicted wound image, or “leader”—what the wound would look like if it healed ideally. The deep reinforcement learning controllers try to “follow” the ideal image by controlling how much treatment is delivered.
The machine learning algorithm only chooses one treatment at a time. Electrical stimulation is applied initially. The treatment automatically switches to drug delivery when the probability that the wound is still in the inflammatory healing stage is 40 percent. (Wounds generally heal in four stages—blood clotting, inflammation, tissue growth, and maturation.)
The bioelectronic actuator used to deliver treatments is a cylindrical silicone polymer body with eight reservoirs arranged in a circle, four for electrical stimulation and four for drug delivery. Each reservoir has an electrode, plus a central counter electrode. A hydrogel connects the electrodes to the wound.
Using iontophoresis, the device delivers either a saline solution for electrical stimulation or a fluoxetine drug solution. “We basically have an electrical current of therapy molecules instead of electrons,” says Rolandi. “Part of the circuit, if you will, is actually the wound bed. By measuring the current, we can count how many molecules go into the wound bed, so we have a very precise control of the dose.”
 Researchers tested the smart bandage—attached to a harness—on a pig.Source images: Houpu Li, Hsin-ya Yang, et al.
Researchers tested the smart bandage—attached to a harness—on a pig.Source images: Houpu Li, Hsin-ya Yang, et al.
Smart Bandage Results
Results showed that 50 percent of the device-treated wound was covered by new skin cells compared to only 20 percent of the control wound. “It’s encouraging that there’s more re-epithelialization,” said Gurtner. The device was used for the first seven days out of a 22 day experiment, but the wounds were not fully closed at 22 days.
One of the genes associated with inflammation, interleukin 1 beta, reduced by 61 percent for the treated wounds. Rolandi says the presence of other genes associated with inflammation or anti-inflammation also trended in the right direction. “Although the [sample size] is low and not statistically significant yet, it’s good to see that epithelialization and the quality of epithelialization, inflammation and the blood vessel growth and wound maturation are consistently showing improvement,” says Zhao.
Overall, “I think it’s a modest effect,” says Gurtner.
The team is hopeful that making device improvements will yield better clinical results. “I think we have a lot of future work to do,” says Rolandi. They published their results in Biomedical Innovations on 23 September.
Gurtner says he would have liked to have seen larger sample sizes and that he “would really like to see them take the wounds all the way to closure with the device on for the whole time, and see what the difference is in magnitude of time to closure.” The device was used for the first seven days out of a 22 day experiment, but the wounds were not fully closed at 22 days.
Rolandi says that the team did not follow the entire healing process because “one, intervening in the very beginning of the healing phase tends to have the most effect on the healing itself as a whole. And two, running these large, animal, preclinical trials becomes very complicated…for this first publication, we decided to start simple and just treat the wound for seven days.”
Moving forward, the team plans to simplify the device. (The current version took them a month to build, says Rolandi) “We are developing a flexible version. That’s certainly something that we knew was necessary.”

























 Researchers tested the smart bandage—attached to a harness—on a pig.Source images:
Researchers tested the smart bandage—attached to a harness—on a pig.Source images: 




